Electronegativity And Polarity Relation
Bond type using electronegativity 6.4 polarity of molecules Chemistry covalent bonds compounds molecular electronegativity difference characteristics ch150 examples diagram
Chemical compound - Trends in the chemical properties of the elements
Electronegativity polarity bond difference type between chemistry two ionic atoms relationship molecular Periodic trends table electronegativity summary chart polarizable electron which most elements list electronegativities presentation ppt trend chemistry radius atom does Stoichiometric basics: chemistry for kids!: electronegativity and
Electronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common
Electronegativity covalent difference bonding elements chloride nacl dummies hydrogen socratic chlorine hcl transition memorize image0 metalsChemistry electronegativity bond polar binding ionic verschil covalente covalent teaching bonding table kids polaire een gradation periodic chemie stoichiometric basics Electronegativity bond type usingElectronegativity and bond polarity.
Polar periodic nonpolar table electronegativity vs molecules electron atom elements when becomes loses below higher darker sourceCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Electronegativity which periodic trends chart polarizable most table summary list trend chemistry elements radius does electronegativities electron presentation energy electronsBond polarity, electronegativity and dipole moment.

Electronegativity polarity bond differences difference atoms polar between electronegative electrons atom if electronegativities ppt covalent powerpoint value bonded
Electronegativity and polar covalent bondingWhich of these are expected to be the most polarizable? Electronegativity polarity bond ppt presentation powerpoint covalent nonpolar slide1Polarity electronegativity bond chemistry.
Electronegativity polarity bondPeriodic electronegativity compound britannica electron values atoms atom cs bonds atomic pairs electronegativities ions nonmetal Polarity molecular shape bond chem polar chemistry chemical libretexts nonpolar electron ionic bonding distributionChemical compound.

Electronegativity (en) & bond polarity
6.1: electronegativity and polarityMakethebrainhappy: polar vs. nonpolar molecules Polarity bond dipole electronegativity moment chemistry practice problems4.3: molecular shape and molecular polarity.
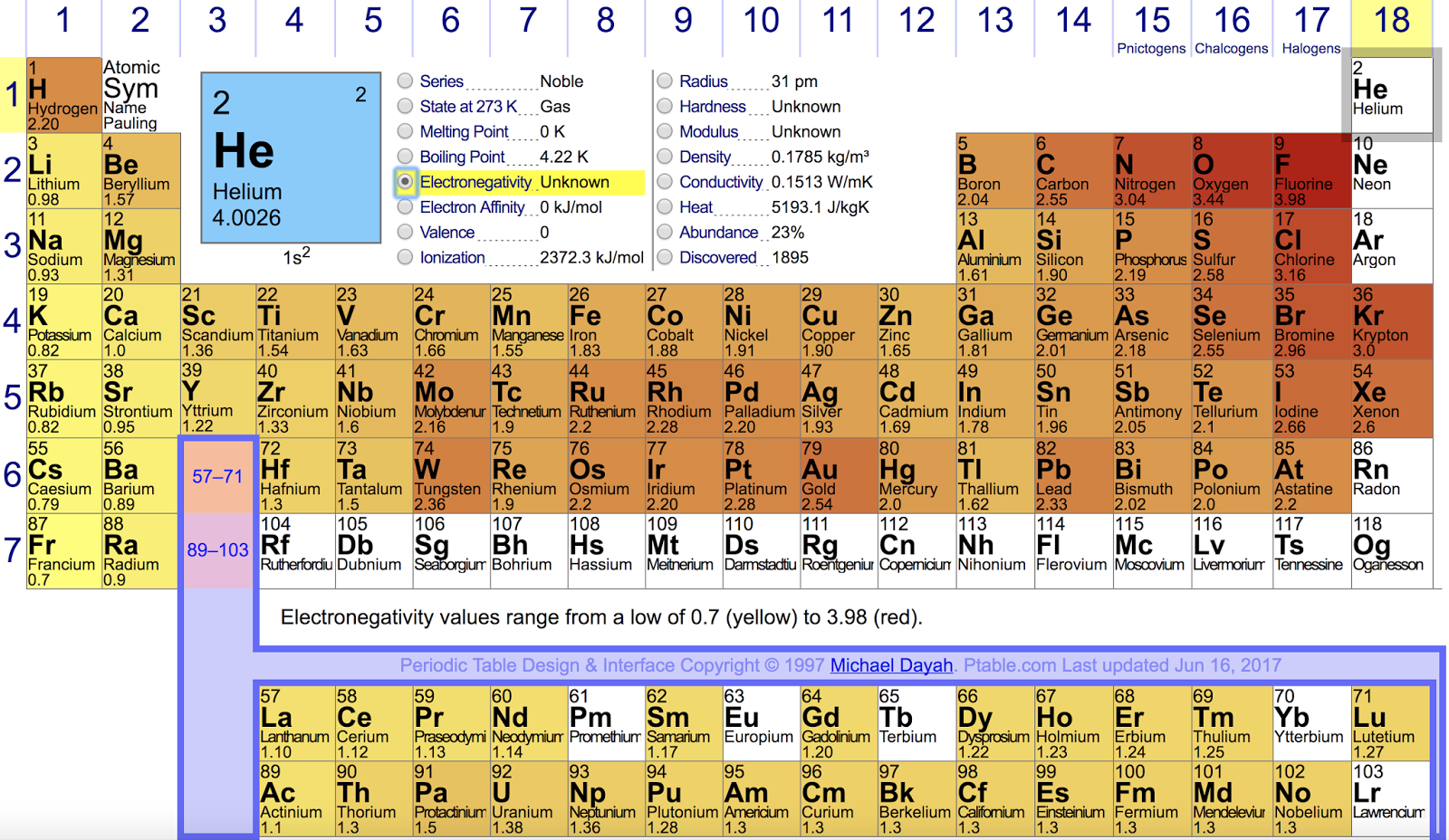
How can i determine bond polarity? + examplePeriodic trends in electronegativity Electronegativity polar difference bond ionic between atoms polarity covalent bonds pure molecules chart type values bonding table vs two increasesElectronegativity chart — list of electronegativity.
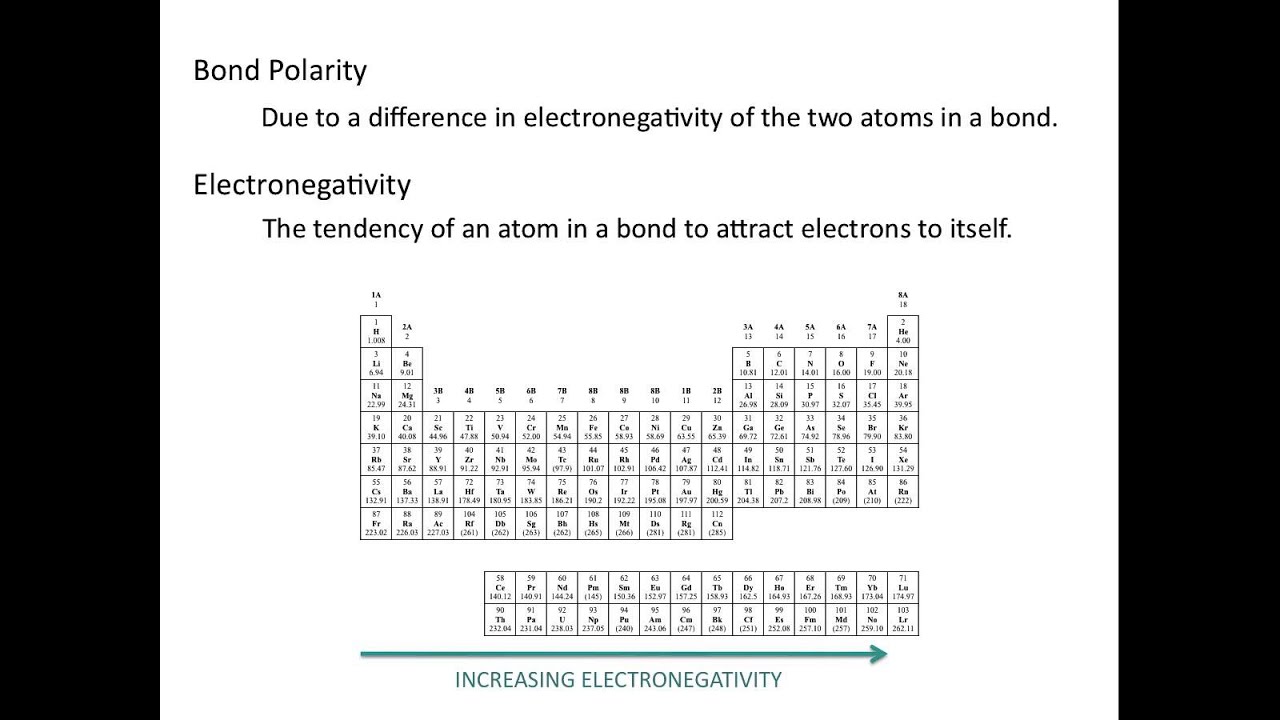
Electronegativity pauling periodic table trends scale values chemistry linus measured period atomic group noble ionic gases chart value printable number
.
.


Which of these are expected to be the most polarizable? | Socratic

Electronegativity - YouTube
MakeTheBrainHappy: Polar vs. Nonpolar Molecules

Stoichiometric Basics: Chemistry for Kids!: Electronegativity and

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

4.3: Molecular Shape and Molecular Polarity - Chemistry LibreTexts

Bond Polarity, Electronegativity and Dipole Moment - Chemistry Practice

Electronegativity and Polar Covalent Bonding - dummies